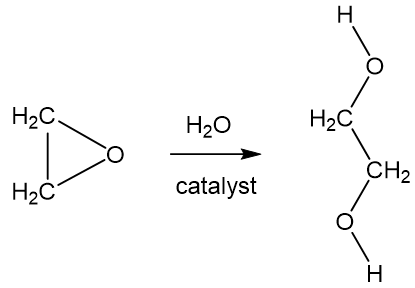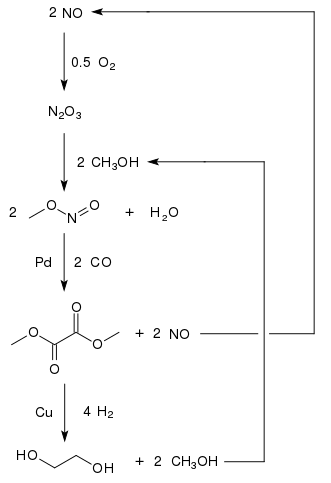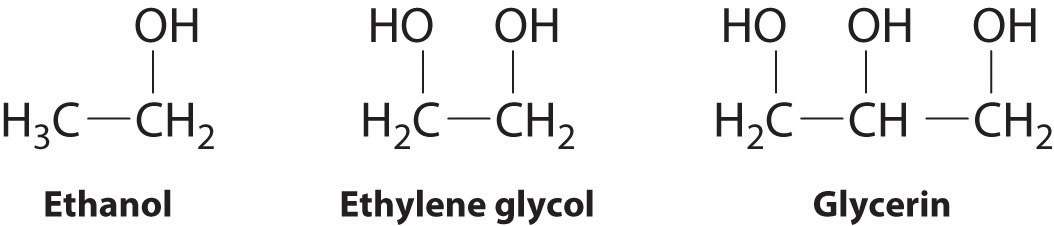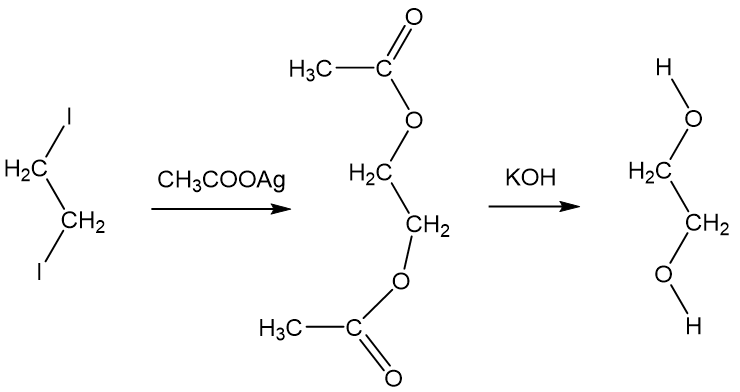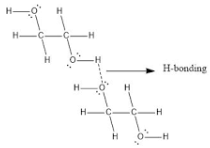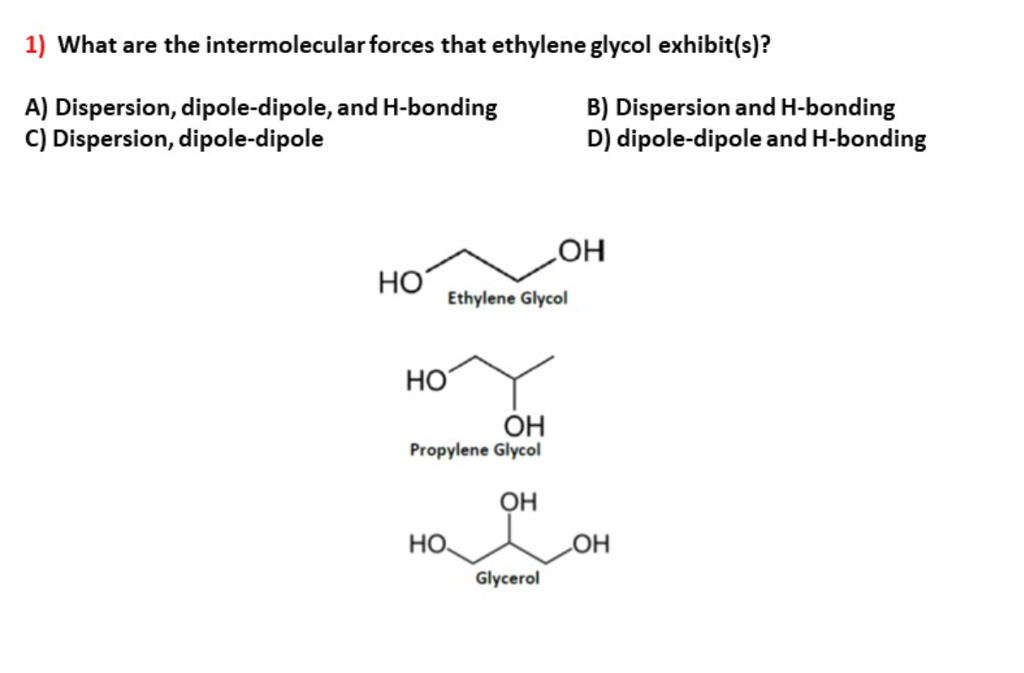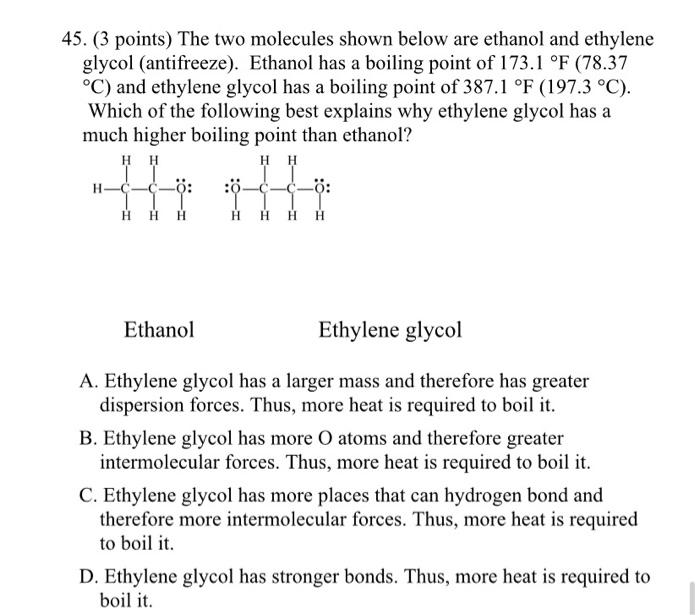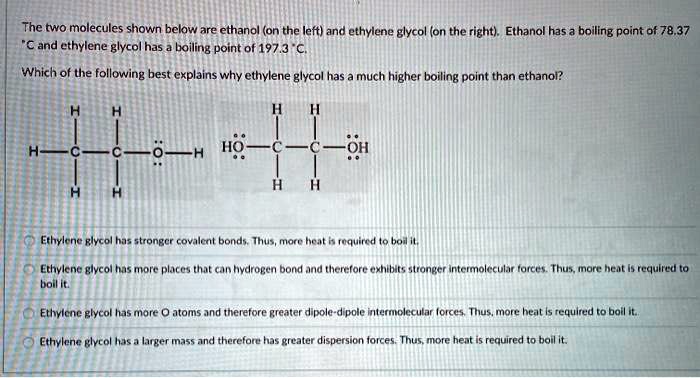
SOLVED: The two molecules shown bclw are ethanol (on the left) and ethylene glycol on the right) Ethanol has a bolling point of 78.37 C and cthylene glycol has boiling point of

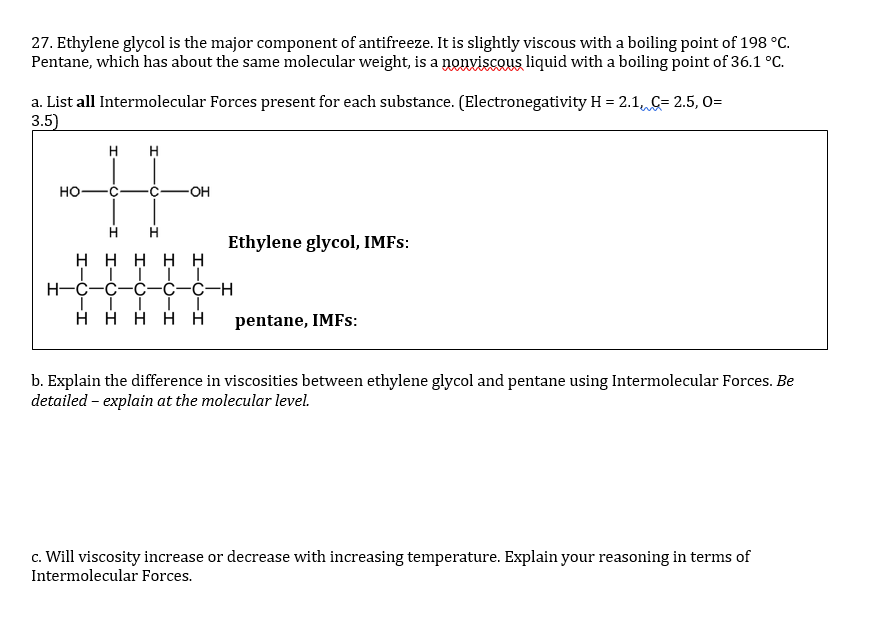
SOLVED: LIQUIDS: Intermolecular Forces Acetone Acetonitrile Nitromethane Compound CzHsO CzHzN CHzNOz (nail polish remover) (used as a solvent) (drag racing fuel) H :0: H :0: Structural H CC-H H C-C==N: HC-N-o: formula

Schematic diagram of the two most stable conformers of (a) ethylene... | Download Scientific Diagram

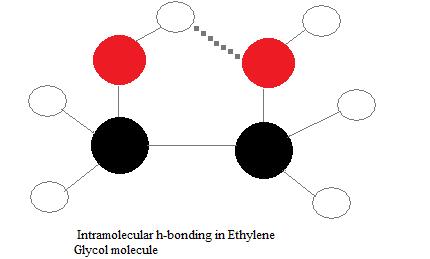
Ethylene glycol, HOCH_2CH_2OH, may look nonpolar when drawn, but an internal hydrogen bond results in an electric dipole moment. Explain. | Homework.Study.com

Contact angles of water, diiodomethane, and ethylene glycol (a) and... | Download Scientific Diagram

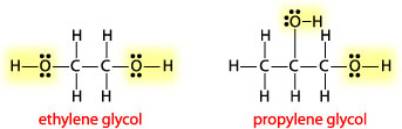
In the Lewis Dot structure of ethylene glycol, would one hydrogen atom visibly connect the two oxygen atoms? And if so, would one line connecting that same hydrogen atom to one of

Ethylene glycol, HOCH_2CH_2OH, may look nonpolar when drawn, but an internal hydrogen bond results in an electric dipole moment. Explain. | Homework.Study.com